CT:IQ has now launched the preliminary outputs for Tools for Flexible Trial Delivery
BACKGROUND
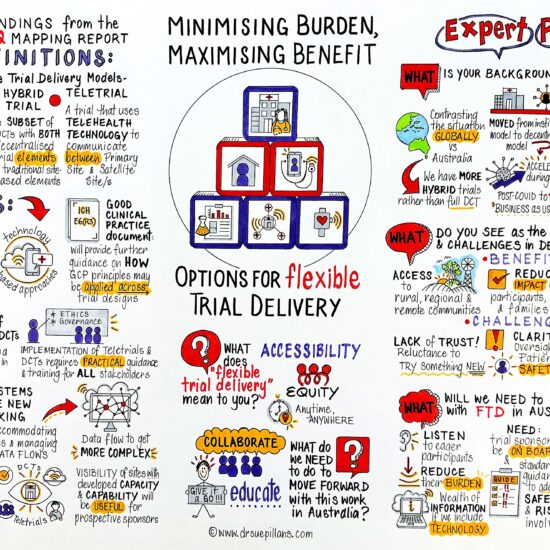
In Australia and internationally, attention has started to focus on opportunities to centre the delivery of clinical trial activities on trial participants rather than clinical research sites.[1] In Australia, considerable funding and effort has been given the teletrial model for decentralisation, being a trial that:
uses telehealth technology to communicate between the Primary Site and Satellite Site/s for some or all aspects of a clinical trial. This supports a Principal Investigator (PI) to supervise Associate Investigators who conduct a clinical trial at a Satellite Site, geographically remote from the PI’s Primary Site.
CT:IQ has commenced the flexible trial delivery models project to explore additional models for centring the delivery of trial activities on participants including delivery at participant homes, in community venues, at non-research healthcare facilities (e.g., pathology laboratories), and through digital platforms.
SCOPE
This project will identify and develop resources to support flexible models of trial delivery. This may include—among other tools—guidance (e.g., on regulatory requirements), templates (e.g., letters of agreement), and case studies of successful implementation of flexible models of trial delivery. The project will incorporate consumer views on flexible trial delivery methods, including their perspectives on respective burdens and benefits of different delivery approaches.
ISSUE
Flexible trial delivery methods can offer opportunities for people to participate in research in less burdensome ways, including closer to home and at times that suit their schedule. This may increase participation as well as participant diversity.[2] Yet, at present, there is no central, national repository of information of flexible trial delivery methods or guidance for their use.
OBJECTIVES
This project seeks to promote a clinical trials environment in Australia that is proactively equipped to implement flexible trial delivery methods. By providing targeted and practical information to the Australian clinical research sector, this project will:
- Make available information about opportunities for flexible trial delivery, to promote consideration and uptake in Australian clinical research.
- Guide practical, effective, and lawful design of protocols incorporating flexible trial delivery methods, including as guided by consumer views.
- Facilitate risk-proportionate review protocols incorporating flexible trial delivery methods by Human Research Ethics Committees (HRECs), researcher institutions, and funders.
PROJECT TEAM
Meet the team driving the Flexible Trial Delivery Project. View the full list of project members and their roles.
[1] Yared Santa-Ana-Tellez et al., ‘Decentralised, Patient-Centric, Site-Less, Virtual, and Digital Clinical Trials? From Confusion to Consensus’, Drug Discovery Today 28, no. 4 (1 April 2023): 103520, https://doi.org/10.1016/j.drudis.2023.103520. [2] R. Donald Harvey and others, ‘A Call to Action to Advance Patient-Focused and Decentralized Clinical Trials’, Cancer, n/a.n/a <https://doi.org/10.1002/cncr.35145>.
Preliminary results released
Access Tools for Flexible Trial Delivery
Publications
Workshop
CT:IQ formally commenced the project with an in-person workshop in Melbourne.
Webinar
Watch our webinar on Flexible Trial Delivery, as experts explored the drivers, benefits, and challenges of flexible trial delivery options in Australia.
Artwork by Dr Sue Pillans